Exploring Clinical Trial Terminology:
Subject, Volunteer, Participant, or Patient?
By Vanessa Peña, Marketing Executive, Clariness
Throughout the years, various terms have been used to refer to individuals who participate in clinical research studies. When considering the most appropriate term for these individuals, it is crucial to select the one that accurately portrays the individual’s role, while acknowledging their sacrifice and contribution, as well as respecting them and their family. However, it is important to assess whether any of these terms misrepresent or disrespect the individual.
It’s important to keep in mind that the choice of terminology in clinical research can be influenced by specific contexts, study designs, and ethical considerations. However, from a general perspective, we can discuss the potential implications of each term, the four most used being Subject, Patient, Participant, and Volunteer.
As advocates for inclusive imagery and language, we at Clariness conducted a survey in 7 countries (South Africa, United States, Canada, Germany, United Kingdom, Poland and Korea) with 300 participants completing the survey. Dedicated to exploring the dynamic landscape of clinical studies and fostering a deeper understanding of the patient preferences for materials, the survey covered:
- Image preferences
e.g. showing the condition or not, animated or live action - Preferred colors
e.g. what emotions are triggered, does the indication have a suited color - Terminology and verbiage
e.g. Technical vs. non-technical terms, preferred ‘labels’
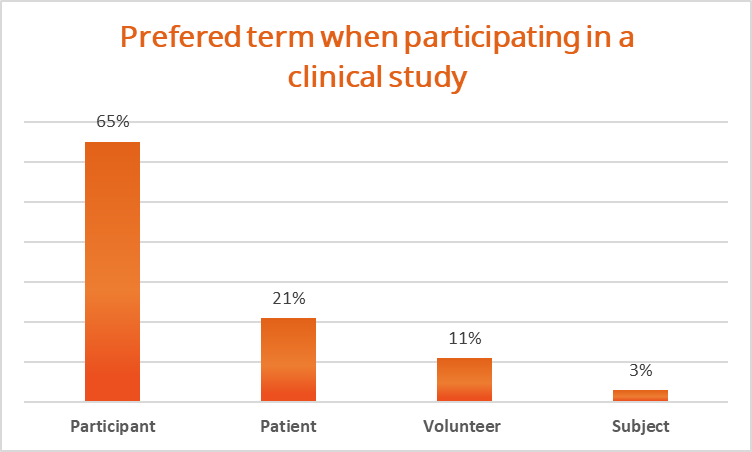
While exploring patient preferences regarding terminology, we asked respondents their preferred term as a clinical trial participant, and here’s what we found:
Definitions of these terms
To establish a clear understanding, we define and clarify the four terms commonly used in clinical trials:
Subject
It is a person recruited to participate in the Clinical Trial or any person who is enrolled in the Clinical Trial. According to the FDA (Food and Drug Administration), a subject is an individual who participates in a clinical trial either as a recipient of the investigational product(s) or as a control.
Patient
The traditional term ‘patient’ is used to represent people who are in receipt of health services. A patient typically refers to an individual who is receiving medical or healthcare services. They are individuals who seek medical attention or treatment due to an illness, injury, or any other medical condition. They are under the care and supervision of healthcare professionals, such as doctors, nurses, or therapists, who provide diagnosis, treatment, and management of their health conditions.
Volunteer
Volunteers are defined as an integral part of the research process. People with a particular disease as well as healthy people both can play a role in contributing to medical advances. Without volunteers, clinical studies simply would not be possible.
Participant
This term works well for those types of research (such as controlled trials) in which active involvement of the people being studied is required.
What do these results mean?
A resounding 65% of respondents expressed a preference for being referred to as “participants” in the context of clinical studies. This finding reflects a growing emphasis on the individual’s empowerment and active engagement in their healthcare journey.
21% preferred the term “patient,” emphasizing the importance of medical context and care within the study setting.
Additionally, 11% expressed a preference for being called “volunteers,” suggesting a willingness to contribute their time and effort to the study’s objectives.
On the other hand, only a small percentage, 3%, indicated a preference for the term “subject,” which may imply a perception of passivity or detachment.
Interestingly, when specifically asked about the term “patient,” most survey participants (61%) expressed either a positive or neutral sentiment. This suggests that the term holds a degree of resonance and familiarity within the medical community.
How to leverage these findings
With these valuable insights, we propose the thoughtful utilization of language in recruitment and retention efforts:
- In patient recruitment materials, the term “patient” may be more suitable, harnessing its connection to the broader healthcare context.
- In patient retention materials, the term “participant” can be employed to emphasize the active role individuals play in advancing medical knowledge.
These findings highlight the diverse perspectives and preferences among individuals when discussing participation in medical research. By understanding these preferences, we can tailor our communication and messaging to ensure clarity and resonance with a wider audience.
In conclusion, the results of these surveys highlight the significance of language in clinical studies and the importance of tailoring communication to meet the preferences and expectations of participants. By adopting these nuanced approaches, we aim to create a welcoming and inclusive environment that resonates with the diverse needs and preferences of those participating in clinical studies.
Want more patient insights to drive your recruitment?
Clariness has 18+ years of experience in supporting 1,200+ international studies and has worked with millions of patients globally to understand which imagery, language, and more have the best conversion rates to ensure you meet your enrollment targets, with the best possible ROI for your recruitment budget.