Boehringer Ingelheim RCV & Clariness expand partnership to bring 20+ studies to additional markets
Clariness, Berlin, November 29 2023 – Today, Clariness announces the expansion of its partnership with Boehringer Ingelheim RCV GmbH, listing 20+ studies in Hungary and the Czech Republic. This marks the first expansion of Clariness’ Registry service into these markets, hosted in its global patient platform, ClinLife®.
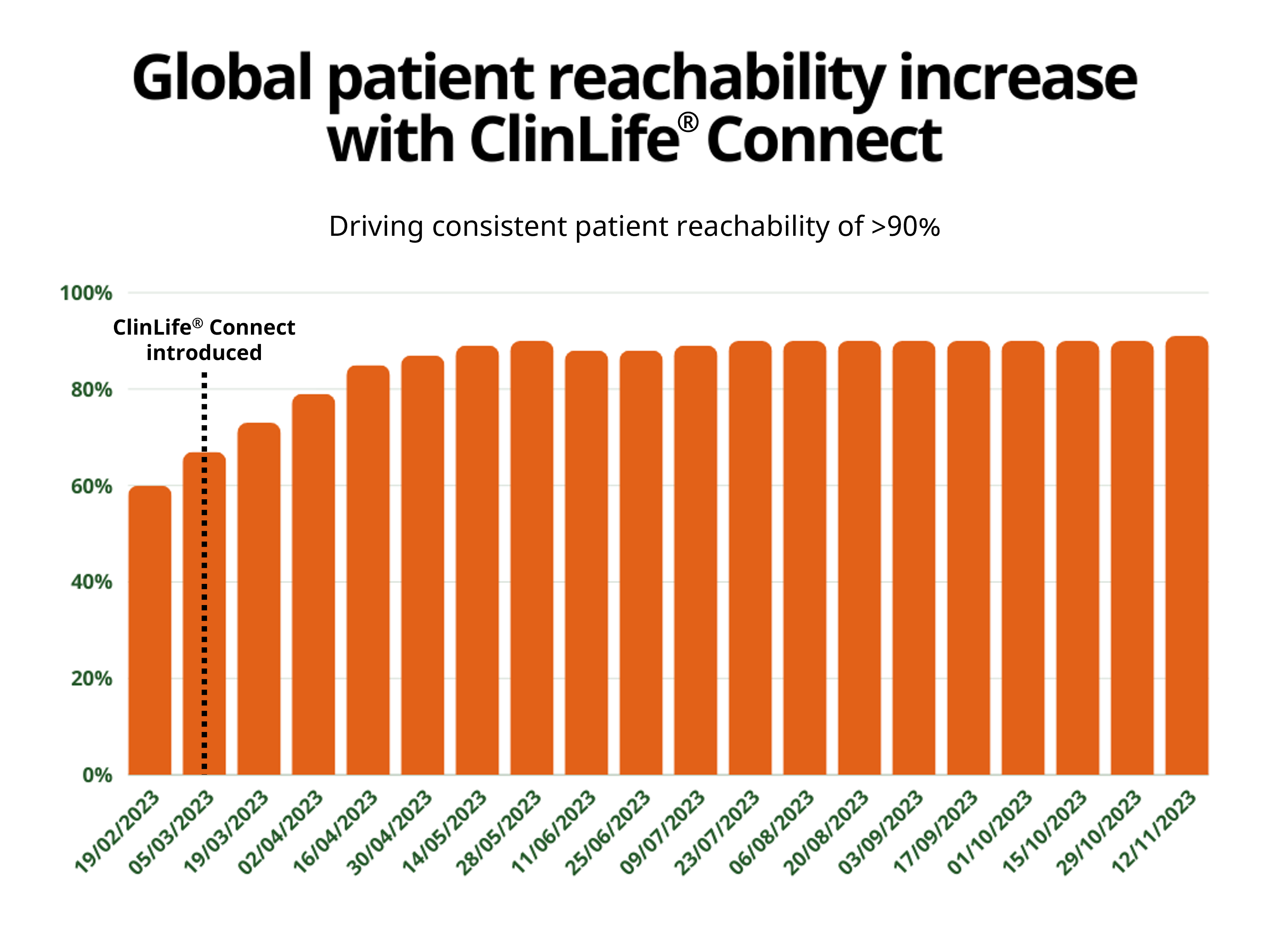
The Registry service uses pre-approved study materials, and digitally advertises, screens and refers patients using indication-specific materials. This innovative approach enables set-up and recruitment is to begin within 2 weeks, routing pre-qualified patient referrals to sites according to patient preference and investigator capacities, and offers the ability to adapt campaign materials and media budget investment at will.
Herman Novak, regional Therapeutic Area Lead in Austria at Boehringer Ingelheim said, “ClinLife® is allowing us to connect more patients, with more studies, using innovative digital recruitment and referral processes. Clariness provides us with transparent reporting on campaigns, channels and site performance, which coupled with the adaptiveness of indication materials and media budget, has allowed us to increase referrals and site screening productivity.”
The studies launched on ClinLife® Registry cover a multitude of indications, including depression, obesity, interstitial lung disease, bronchitis, Netherton syndrome, and more. The launch of these studies in Hungary and the Czech Republic marks the second international expansion with Boehringer Ingelheim on ClinLife® Registry, following the 2021 expansion to Poland, Austria and Switzerland.
Moritz Kloss, Vice President of ClinLife® at Clariness, said “We are very excited to expand our partnership with Boehringer Ingelheim to support these additional studies in new countries. Over the last few years, our ClinLife® Registry service has fulfilled all expectations, evidenced through bringing this service to 5 new markets with Boehringer Ingelheim.”
About Clariness
Clariness provides high-end services across the clinical trial lifecycle, including study feasibility, patient recruitment, and patient retention services, for pharmaceutical companies, contract research organizations (CROs), and study sites across the globe. Dedicated to improving patients’ lives by accelerating the development of medical treatments and therapies, Clariness is the leading expert in global patient recruitment with 18+ years’ experience. Clariness has supported over 1,200 clinical trials worldwide through its market leading patient portal ClinLife®, which is active in 50+ countries, available in 35+ languages, covers 80+ indications, and welcomes 15+ million visitors per year. For more information, visit www.clariness.com and www.clinlife.com.
About ClinLife®
ClinLife® is a patient-friendly digital platform that allows users to browse indications, find matching clinical studies and apply for them via the portal. Different sponsors, sites, CROs and digital health applications list a single study or an entire portfolio of clinical trials on ClinLife®.
About Boehringer Ingelheim RCV
Boehringer Ingelheim RCV is working on breakthrough therapies that transform lives, today and for generations to come. As a leading research-driven biopharmaceutical company, it creates value through innovation in areas of high unmet medical need. More than 52,000 employees serve over 130 markets in the three business areas, Human Pharma, Animal Health and Biopharmaceutical Contract Manufacturing. For more information, visit www.boehringer-ingelheim.com.
Press contact: Joseph Yeomans, VP Marketing, [email protected]