Successful clinical trials begin with a conversation between patients and sites that help them feel comfortable, supported, and informed. Effective recruitment and retention materials for both patients and HCPs are vital to making this conversation possible.
Clariness’ study creatives provides a way to simplify complex protocol information, boost study awareness, and strengthen patient confidence throughout their clinical trial journey. This FAQ highlights some common questions sponsors have as they work with us to optimize their enrollment, improve participant engagement, and build better recruitment and retention strategies for both HCPs and patients.
What type of videos do we make to support patient recruitment and why do they matter?
At Clariness, we create animated videos that describe to patients, potential participants, and caregivers what the study is about, what is a clinical study, and what can be expected during a study. These videos help to increase clarity and knowledge about indications and the study, which can be a comfort to potential participants. They may be more willing to participate in the study if they have a clear understanding of how exactly the clinical study will be done as well as how the study medicine or treatments work.

Which trial creatives work best for recruitment and retention?
While full packages are usually created for studies, the most common retention materials that are requested include:
- Welcome guides
- Appointment reminder cards
- Websites
- Videos
- Thank you cards
Welcome guides help participants stay on track with their study visits and have a clear picture of what is upcoming with each visit. The more clarity a participant has, the more comfortable they feel about continuing with the study. Also, appointment reminder cards ensure that the date and time is written on the card so that participants know exactly when and where they are expected, as well as what assessments and procedures they will have.

Why are thank you cards important in clinical trials?
Thank you cards and milestone cards are two of the most valuable assets of patient retention. If patients feel unsure about staying in a clinical study, receiving a card that reminds them of their participation matters can help them feel more committed to continue. It is a small, but mighty way to show gratitude and care towards the time, effort, and trust that participants give throughout a clinical study. These thank you or milestone cards could also be utilized as digital assets such as emails or push notifications on trial platforms.

How to design creatives for pediatric patients?
Communicating with pediatric patient populations requires an especially thoughtful balance between clarity, emotional support, and respect for the children and their caregivers. Since medical terms are often overwhelming and intimidating to children, we translate them into child-friendly explanations with simple sentences, calming word choices, gentle reassurances, and relatable comparisons.
We also tailor the images and designs to the age groups in the trial, using illustrated characters, friendly diagrams, and storytelling. We include activities like coloring, small puzzles, or games in welcome guides or other materials so that children can also have something to enjoy.
Another important aspect of creatives for pediatric patients is also emphasizing that the care of the child comes first. We can address questions plainly like, “Will it hurt?”, “Who will be with me?”, or “Can I bring something from home?”. Caregivers also receive detailed information aligned with the child-facing materials with explanations of procedures, guidance for supporting their children, and reinforcement of safety and oversight.

How do we tailor messaging and imagery for caregivers?
Clariness knows how essential caregivers are to all clinical trials, and we write materials for them with clarity, confidence, and practical guidance in mind. Caregivers often have to balance making medical decisions, daily care and responsibilities, as well as emotional pressure. We ensure all of our caregiver materials validate their experience, recognize the challenges they face, and give appreciation to them for their vital contribution throughout the clinical trial.
For caregiver materials, it is also especially important to include safety, logistical, and time management information. We address concerns such as how to prepare their loved one for study visits, how long appointments may take, and potential risks that should be monitored.
What is a study identity? Is it really that important for my trial? (More than a logo, it’s everything)
For cohesion throughout the study, to give clarity, and to craft a better story, a study identity is a must for a clinical trial so patients can visualize their journey. Study identities are essential to ensuring that patients feel emotional recognition to the trial materials and also find a clear and consistent message across a wide range of assets. This recognition and relatability can only be reached with a comprehensive study identity.
Does the study identity need EC/IRB approval?
Yes, the study identity does need EC/IRB approval as it also contains images and graphics that will be used throughout the trial materials. This is taken into consideration when we create our timelines for the study identity.
Why do we need a name for the logo?
The clinical trial name gives the study its identity since the protocol title is too scientific and unemotional for patients to connect to. A study name summarizes in one word to people about something that they can emotionally relate to. A name also allows participants to feel more personified when they discuss their involvement in the study with either their doctors or loved ones. Then, by creating a logo with the study name for a clinical trial, the logo reflects the meaning of the name and makes it more impactful.
How important is a study protocol for the creative process?
A finalized study protocol is essential for developing the study’s creative materials. Without a complete, approved study protocol, the creatives team may not have all the information necessary to make proper retention and recruitment materials. We also run the risk of creating items that will need to be changed soon due to protocol amendments or protocol updates due to beginning creation with draft protocols.
What is the average lead time for developing a complete patient recruitment toolkit?
The lead time for a recruitment package varies depending on the amount of materials requested, but for a full patient recruitment toolkit we advise it takes an average of about 2 months. This includes the creation of the study identity, revisions of the content, and also layout implementation. This does not include translations or production time.
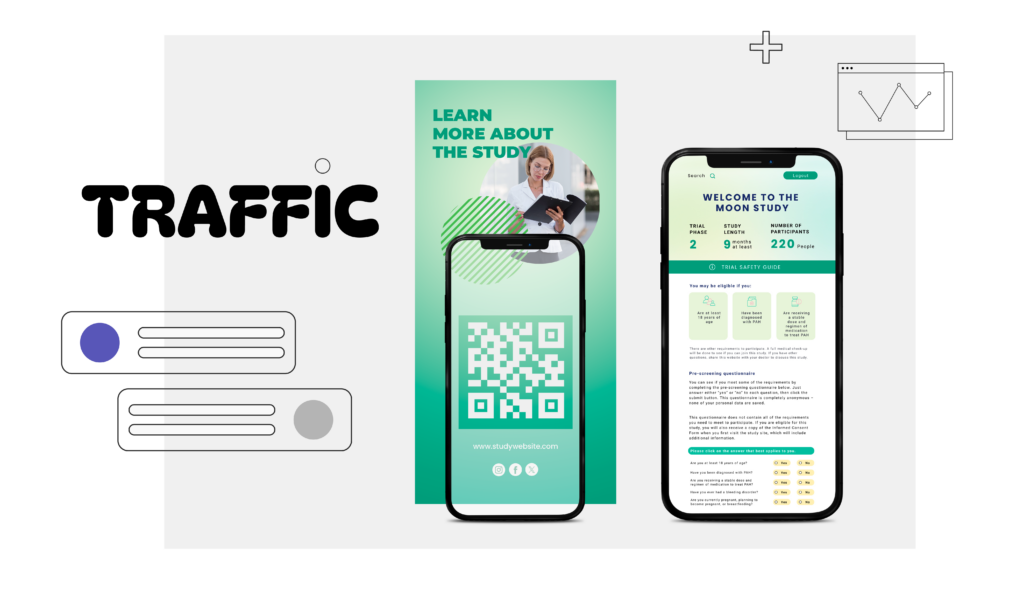
How do creatives drive trial website traffic?
Creative materials help guide potential participants to the trial website through several channels. Many clinical trial assets include QR codes that lead users directly to the site, embedded links placed throughout printed and digital documents. When QR codes are combined with UTM tagged links, it becomes possible to clearly track where website traffic originates, whether from offline or online materials.
Another effective way to increase traffic is through Search Engine Advertising (SEA). SEA campaigns target specific keywords and search phrases on Google, allowing the campaign to reach the relevant audience and bring additional qualified visits to the trial website.
In Conclusion
Building a strong connection with patients requires thoughtful communication, a clear clinical trial identity, and a deep understanding of all those who will be involved in the clinical trial, from participants to caregivers and HCPs. When recruitment and retention materials are designed with intention, they do more than enrollment support. With clarity, empathy, and consistency, sponsors can help create trial experiences that not only meet recruitment goals but also honor the patient’s medical journey.
With over 20 years of experience in clinical trial patient recruitment, Clariness has developed (printed & shipped) over 48,000 creative assets to support 1300+ study sites. Our clinical trial branding materials have been used in 40+ countries worldwide and across 50+ indications.
No matter the therapeutic area, geography, or site needs, Clariness delivers branded clinical trial materials that are compliant, consistent, and built to perform.
Ready to streamline your next study’s creative development? Let’s talk about your branding needs.
Author: Jackie Janssen, Medical Copywriter/Content Creator Clinical Trials | Post Date: 16.01.2026