Asthma patient recruitment for clinical trials
Need help accelerating your study?
Asthma is a global condition affecting approximately 300,000 million individuals worldwide – with cases expected to increase another 100 million by 2025. While there is currently no cure, clinical trials can help patients with asthma lead a more normal and active life.
We’ve performed asthma patient recruitment for 30+ international trials, from Phase I to Phase III – we know how to find the right candidates for your study.
Special screening techniques
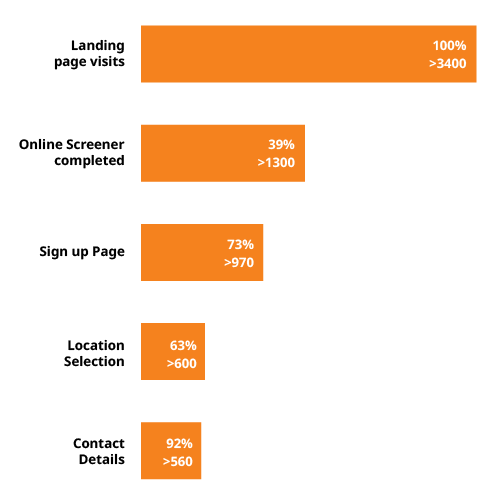
Our targeting techniques drive high volumes of traffic to our customized screeners, leading to higher numbers of referred and randomized patients for asthma studies. For example, one campaign drove 900k+ visits to our ClinLife trial-specific landing pages.
Given varying epidemiological definitions of asthma, we develop personalized screeners for your study consisting of multiple questions assessing medical eligibility, including I/E criteria, diagnosis, and symptoms.
Ensuring a high scheduling rate, we also provide a customized phone screener that covers participation willingness and a detailed assessment of medication history. This helps to limit study delays and ensures more consented and randomized patients.

Find patients aligned to your protocol
Asthma is a heterogeneous condition presenting with many phenotypes. Importantly, age of onset appears to play a key role in patient health. Although asthma is among the most common chronic diseases in children, adults with asthma experience higher morbidity and mortality than children.
We are able to actively reach both children and adults for clinical trials. For example, the youngest patient enrolled in a trial was 4 years old, while the oldest was 80 years old.
Our Chief Medical Officer reviews your protocol and aligns with our patient recruitment team. We develop and create objectives and tactics to ensure that patients referred to your sites fit the exact requirements of your study.

Leveraging decentralized solutions in your asthma studies
Embracing new decentralized clinical trial (DCT) solutions can help your clinical trial stay on track and reduce patient drop-out. For example, remote sensing devices enables continuous real-world data collection of asthma symptoms and asthma attacks, while electronic patient diaries allows patients to easily record their daily symptoms.
We can help you adopt new approaches and transform your trial by navigating regulatory changes, creating study materials for patients and caregivers to better understand new technologies, and providing intensified support to sites to reduce site burden.
Our experience with hybrid and DCTs can be applied directly to your study.



