Patient recruitment for Alzheimer’s disease clinical trials
Connect with Alzheimer’s patients across the globe
Globally, almost 10 million new cases of dementia are diagnosed every year, with Alzheimer’s disease accounting for 60-70% of cases.
While the number of diagnosed patients continues to grow, clinical trials on Alzheimer’s disease in particular are facing a critical shortfall of eligible patients. We’ve performed Alzheimer’s disease patient recruitment for 10+ international trials, from Phase II to Phase III – we know how to find the right candidates for your study.

Reach patients and caregivers across the globe
Those within the early stages of Alzheimer’s disease wish to hold onto their independence for as long as possible, while those further along require additional care and are more difficult to reach and engage.
That’s why at Clariness, we target both patients and caregivers, which provides much higher engagement and participation willingness. In fact, within a recent survey of 600 participants across 21 countries, 85% of respondents were caregivers.
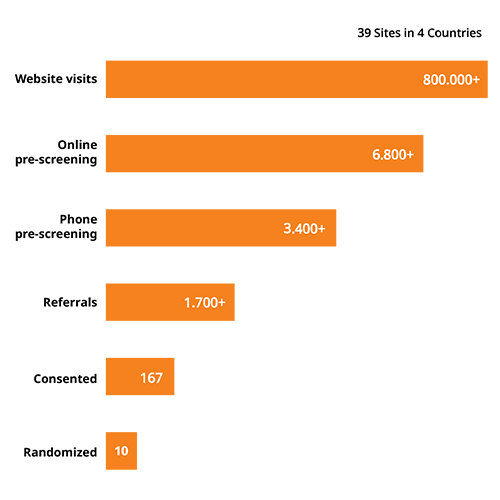
Special screening techniques
Our targeting techniques drive high volumes of traffic to our customized screeners, leading to higher numbers of referred and randomized patients for Alzheimer’s disease studies.
For example, one campaign drove 800k+ visits to our screener that consisted of multiple questions assessing medical eligibility, including I/E criteria, diagnosis, and symptoms.
Ensuring a high scheduling rate, we also provide a customized phone screener that covers participation willingness and a detailed assessment of medication history. This helps to limit study delays and ensures more consented and randomized patients.
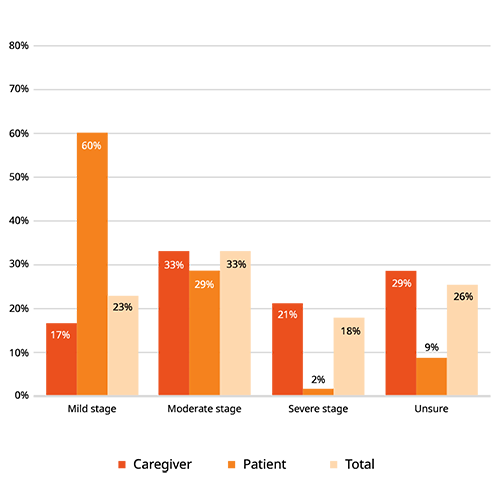
Find patients aligned to your protocol
Most patients with Alzheimer’s disease are 65+ years old – although early-onset Alzheimer’s can develop earlier.
We reach patients with mild to severe stages of Alzheimer’s disease, as well as their caregivers. We target these patients at stages within their lives where Alzheimer’s is most common.
Our Chief Medical Officer reviews your protocol and aligns with our patient recruitment team. We develop and create objectives and tactics to ensure that patients referred to your sites fit the exact requirements of your study.

Addressing patient diversity
Historically, marginalized populations are underrepresented in Alzheimer’s disease clinical trials – despite evidence that these populations may be at increased risk of developing dementia. These include patients that identify as racial or ethnic minorities and those with low socioeconomic status or are living in rural areas.
Informed by Patient Insights, our Alzheimer’s disease patient recruitment and retention services allow us to reach a more diverse population. We use digital tools and multiple communication channels to reduce physical barriers to site access, create patient-friendly study materials to increase health literacy, and tailor strategies to local communities and cultures.
By understanding patient needs, we can overcome the unique barriers these patients face when joining clinical trials.

Find patients aligned to your protocol
Embracing new decentralized clinical trial (DCT) solutions in your clinical trial improves the diagnosis and assessment of Alzheimer’s disease.
Recent research shows that wearables, unobtrusive sensors, or other digital technologies enables continuous real-world data collection that captures subtle changes in cognition and functional capacity of patients. These technologies improve trial timelines by better identifying suitable patients for trials (at earlier stages of their Alzheimer’s disease) and improving assessment of disease progression and treatment response throughout the trial. Digital technologies also enhance patient care and safety, while providing caregivers with an additional tool to look after their patients.
We can help you adopt new approaches and transform your trial by, for example, navigating regulatory changes, creating study materials for patients and caregivers to better understand new technologies, and providing intensified support to sites to reduce site burden.
Our experience with hybrid and DCTs can be applied directly to your study.
Are you designing an Alzheimer’s disease study, or have one underway?
We can help you find the right patients, at the right time. To find out more, contact us.



