Why designing a modern study protocol can save your clinical trial
There is a growing interest in therapeutic advancements in genomics medicine, orphan drugs, and precision medicine. This interest has driven the need to generate robust clinical findings that can set a momentum for stakeholder acceptance of newly approved treatments. Yet many clinical trials continue struggle to generate quality trial results as they deal with clinical development inefficiencies and ballooning costs.
90% of clinical trials reportedly fail to meet patient enrollment goals, leading to delays in treatment development.1
Pharma companies must consider whether they are doing enough to save their clinical studies from failure by conducting a clinical trial feasibility assessment. The traditional way of conducting clinical studies clearly no longer serves the study requirements of today, and pharma companies must seek modern solutions.
Why does your study need saving?
There are three key reasons your clinical study needs saving from traditional approaches:
- Increasing protocol complexity
- Recruitment challenges
- Budget constraints
Traditional in-house capabilities are unable to address complex study protocols.
Traditionally, drug development managers are placed in charge of developing trial design while clinical research managers are tasked with recruiting site investigators and patients. While managing studies in-house has worked in the past, this no longer serves pharma companies of the 21st century due to the increased protocol complexity of clinical trials. Today, patient profiling must be more precise, procedures need to be more standardized, and the viability of indications must be clear.
Study protocol complexity has also contributed to the challenge of effective site selection. Indeed, more than 80% of trials in investigative sites fail to meet their patient enrollment deadline, and 30% of Phase 3 trials are terminated due to enrollment and protocol difficulties.2 To deal with complex study protocols, pharma companies require a modern team – one that’s dedicated to, and experienced in, clinical trial recruitment planning, study feasibility assessments, and strategic patient retention.
Traditional patient enrollment methods are problematic.
Many investigators struggle with poor patient enrollment and low retention, which can lead to a lack of quality patient input. One reason for this struggle is that investigators are competing for participants. Clinical trials have increased in numbers over the past 20 years, from 2,119 studies in 2000 to more than 280,000 studies in 2018.3 However, the number of participants in clinical trials has decreased from more than 105,800 in 2015 to less than 26,000 in 2016.
With a decreasing number of participants, the need to look to other ways of patient recruitment remains essential. Considering pharma companies are not designed to be clinical trial recruiters, it is possible for them to miss certain gaps. For example, patient populations requiring additional attention and empathy throughout the trial process may not be fully addressed during in-house studies. Specifically, the general patient population is aging and there is an increasing need to represent elderly patients in clinical trials. However, elderly patients are typically underrepresented in studies.4 For studies that involve patients with mobility issues or difficulty with access to investigation sites, pharma companies may also struggle to recruit and gather patient data as these patients might have limited ability or interest to visit a clinical site.5
Traditional study protocols are overwhelming sponsor budgets.
Sponsors can invest more time and money into re-doing the clinical recruitment process until the right patient profiles are located and in-depth results are attained from in-house studies. However, this process is not cost effective and any delay in recruitment leads to additional costs that could be detrimental to business. For example, companies developing small medical products reportedly lose up to $600,000 in foregone sales for each day of delay. Companies need to be savvy enough to cut delays immediately, as drug development costs continue to increase.6 This means pharma companies need to maximize every cent of their budget.
How can a modern study protocol help?
A solution to saving your study is embracing a modern study protocol. Four components of a modern study protocol are:
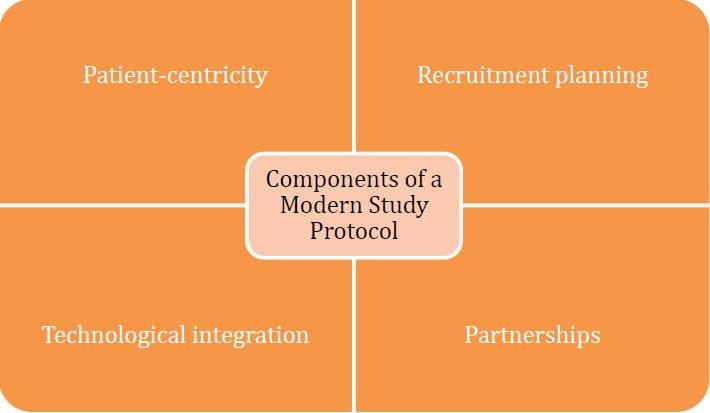
Embracing patient-centricity
Saving your study by adopting a modern study protocol first requires a change in mindset. Sponsors need to understand the importance of applying patient-centricity in treatment development – it is an overarching theme in clinical studies that sponsors need to revisit throughout treatment development. The needs of patients can change over the period of clinical development and along the patient journey so having a deep understanding of patients is key to meeting their needs consistently and effectively. To build that understanding, sponsors must listen to patients, identify their concerns and unmet needs, and view diseases from the patient perspective. With a patient-centric view, sponsors and investigators can better map out trial design, ensuring that the objectives of the study align with the needs of the target patient profile. This approach to trial design can reduce burden on the patient, promoting their well-being, comfort, and convenience, as well as facilitating patient outcomes.
Rigorous patient recruitment planning
One key application of patient-centricity in clinical studies is in recruitment. The recruitment process in a modern study protocol is intended not only to locate patients but also to build relationships with them while considering their needs each step of the way.7
The recruitment process in a modern study is clear, rigorous, and effective. It constitutes a robust overview of the target patient profile and how participation in the study is marketed to each patient. It is designed in a comprehensive manner such that sponsors have no need to revisit recruitment later during the clinical development process.
Generally, a modern approach to recruitment can:
- Expand patient outreach
- Execute quality pre-screening measures
- Generate patient interest
- Build patient awareness
- Identify and target quality patient profiles
- Facilitate quality patient referrals.
Furthermore, modern recruitment methods adhere to patient recruitment standards and the company’s recruitment policies. The modern recruitment process is flexible in that it can be integrated seamlessly into the broad development program. The evolving development requirements of drug manufacturers are also considered.
Adoption of digital patient recruitment technology
In support of an advanced method for recruitment, a modern study protocol leverages the benefits of technological developments. With the use of social media, mobile applications, and digital marketing techniques, among others, sponsors can adopt a more creative approach to patient outreach programs.
In a modern study, the opportunity to utilize digital advertisements and reach specific personas is maximized. The use of emerging digital and mobile tools can save companies between 30% and 50% in recruitment time by integrating more efficient patient identification and screening methods.8
Digital patient recruitment can also serve as a favorable starting point to drive patient engagement. Digital engagement enables sponsors to gain valuable insights into patients, which can help reduce dropout. Online platforms provide opportunities to strengthen and shape relationships with each patient by providing individualized patient education, support, and reminder mechanisms.9 With digital engagement, participants can also share their health information via mobile platforms, facilitating data gathering while enhancing patient satisfaction.
Partnering up for better results
An effective strategy to save your study from failure is to partner with specialists that can ensure faster and better planning and execution of your study. Given the rising costs and inefficiencies in running in-house clinical studies, contracting out the recruitment process and other aspects of clinical studies has become critical to shaping the competitive advantages of pharma companies. Indeed, many companies have picked up on the trend of outsourcing aspects of clinical trials – an estimated 60% of early phase trials were contracted out in 2017.10 Partnering with the right clinical recruiter can help to accelerate trial completion and get new medicines to market sooner.
Essentially, partnerships fill the gaps within the study that in-house development teams might miss by:
- Offering skills and capabilities to ensure a global reach of patients
- Enabling a quick set-up of recruitment campaigns
- Ensuring timely study completion without need to revisit recruitment
- Focusing efforts on ensuring the study is on schedule
- Harnessing a network of healthcare providers and other stakeholders who can contribute to the quality of the study.
So, although there is a need to generate useable clinical findings while working with a limited budget and time constraints, the integrity of a clinical trial need not be compromised. Achieving this approach is made easier with the right partner that can provide patient, site and sponsor support.
The growing pressures to produce competitive medical products are prompting pharma companies to take on innovation-based and value-driven approaches to treatment development. Indeed, value in clinical studies can best be harnessed by adopting modernized methods to protocol design and as such, the best way to save your clinical trial from failure, involves embracing modern study solutions.
To find out how Clariness can help, contact us here.
References
- Stempel, D. (2017). New Tufts Research Explores Barriers to Clinical Trial Recruitment Success. MD Connect. Available at: https://www.mdconnectinc.com/medical-marketing-insights/tufts-research-barriers-clinical-trial-recruitment-success [Accessed 5th January 2019]
- Shpilberg, S. (2017). Social Media for Clinical Trial Patient Recruitment: The Comprehensive Guide, AMWA Journal, 32(4), 2017, pp. 162-165. Available at: https://c.ymcdn.com/sites/www.amwa.org/resource/resmgr/journal/Spotlight/2017v32n4_SocialMedia.pdf
- Statista (2018). Total number of registered clinical studies worldwide since 2000. Statista. Available at: https://www.statista.com/statistics/732997/number-of-registered-clinical-studies-worldwide/ [Accessed 08 January 2019].
- Pond, E. (2018). Elderly Patients Underrepresented in Clinical Trials for Rheumatoid Arthritis, Osteoarthritis. Rheumatology Advisor. Available at: https://www.rheumatologyadvisor.com/rheumatoid-arthritis/elderly-patients-underrepresented-in-clinical-trials-for-rheumatoid-arthritis-osteoarthritis/article/819219/ [Accessed 07 January 2019].
- Palmer, J. (2018). The Future of Mobility and Medicine. Applied Clinical Trials. Available at: http://www.appliedclinicaltrialsonline.com/future-mobility-and-medicine [Accessed 07 January 2019].
- Sullivan, T. (2018). A Tough Road: Cost to Develop One New Drug Is $2.6 Billion; Approval Rate for Drugs Entering Clinical Development is Less Than 12%. Policy Med. Available at: https://www.policymed.com/2014/12/a-tough-road-cost-to-develop-one-new-drug-is-26-billion-approval-rate-for-drugs-entering-clinical-de.html [Accessed 5th January 2019]
- Clariness (2018). ‘Patient recruitment’. Clariness. Retrieved from: https://clariness.com/patient- recruitment/ [Accessed 8th January 2019]
- Anderson, D., Fox, J. and Elsner, N. (2018). Digital R&DTransforming the future of clinical development. Deloitte. Available at: https://www2.deloitte.com/insights/us/en/industry/life-sciences/digital-research-and-development-clinical-strategy.html [Accessed 6th January 2019]
- Ilancheran, M. (2018). Analyzing the Top Clinical Trial Outsourcing Trends Of 2017. Clinical Leader. Available at: https://www.clinicalleader.com/doc/analyzing-the-top-clinical-trial-outsourcing-trends-of-0001 [Accessed 08 January 2019].
Want to know how we can support your patient recruitment?
Simply get in touch with one of our experts, and we will review your study’s requirements, and develop a strategy to enroll more patients.
Insights and recourses



