The shortage of qualified personnel continues to severely impact clinical research, resulting in delayed trials, poor randomization rates, and even cancelled trials. This shortage is logically closely related to another widespread workplace problem in the broader labor market, namely workload and burnout, which also has an even greater impact on clinical research. In fact, since the start of the pandemic, 76% of healthcare professionals, including staff at clinical research sites, have reported feelings of burnout, compared with little above 50% in other professional fields. [1] With a turnover rate twice as high (32%) and a 9.3% increase in vacancies across the sector, especially for critical positions such as study nurses, the problems will only get worse if nothing is done.
In this blog, we look at sustainable, long-term solutions that clinical trial organizers such as sponsors and CROs, as well as research centers themselves, can take to reduce staff workload and thereby also reduce associated burnout and attrition rates. As we show, this solution not only reduces the workload of site staff, but also has a significant impact on the number of randomizations for a clinical trial.
Interested in other measures that clinical trial organizers can take to reduce clinical trial delays? Please download our free whitepaper. If you want to see directly how we’ve reduced site burden in recent studies, download our site success stories below.
How site staff workload, turnover rates and open vacancies are connected, and lead to clinical trial delays
The statistics below make it clear that both workload, turnover rates, and the difficulties filling open vacancies are significantly higher in clinical research than in other sectors. The journal Health Policy shows that it is especially the patient-facing side of clinical trials that faces this problem, with study nurses quitting or looking for jobs in entirely different sectors due to increasing workload.
Yet, this is not just related to factors as lack of staff or the increased use of decentralized clinical trials that result in increased time spent on remote visits. More so, this ”patient-facing side” of clinical trials, is also the area most likely to be the reason behind clinical trial delays or even termination.
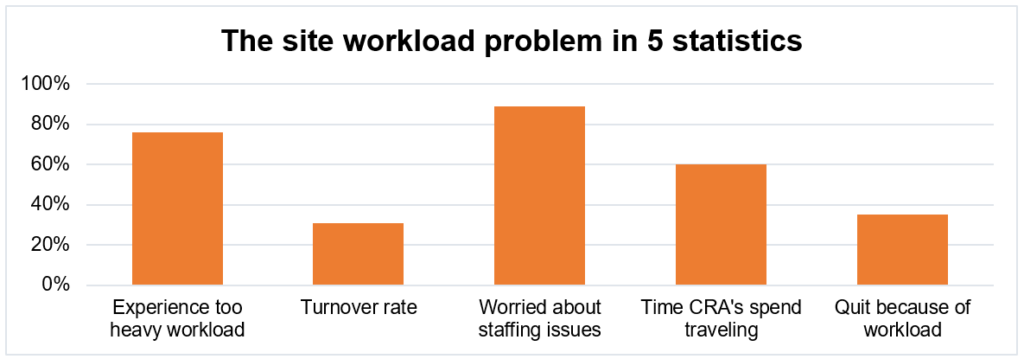
Statistics come from various sources. [2]
This is also highlighted by recent surveys conducted across research sites in the United States and Western-Europe, which show that turnover rates are highest for patient-facing staff, having increased from 10% to 35% before the COVID-19 crisis to current rates of 35% to 61%. [3]
These lead to…
- Increased workload for remaining site staff & lower productivity and effectivity
- Knowledge loss of trial procedures and screening
- Recruitment and training costs
- Bad patient experience, referrals dropped by site, and lower randomization rates
- Higher dropout rate due to lack of (quality of) patient care
The graph below highlights how these workload problems for site staff are directly interconnected and need a comprehensive solution:
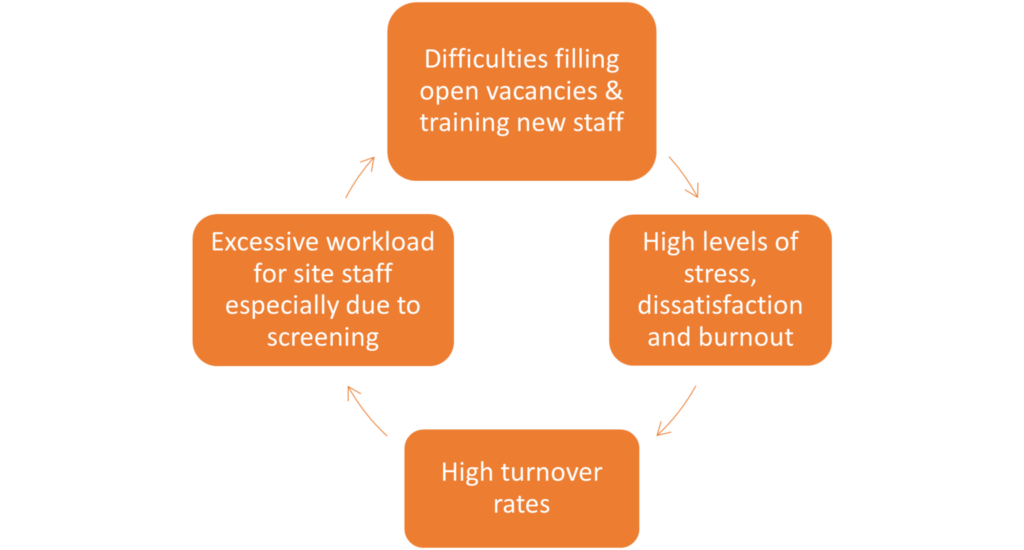
In an open letter to sponsors and CROs this year [4], the Society for Clinical Research Sites said:
“Turnover doesn’t just impact sites – more importantly, it impacts the subjects and their willingness to begin or continue trials. It [impacts] our ability to enroll [and] our ability to retain subjects and to make clinical trials a positive experience.”
Why “simply” improving and speeding-up hiring isn’t the solution
While study organizers no longer treat these issues as an elephant in the room, most surveys and reports indicate that sponsors and sites have presented improved recruitment methods with, for example, sign-up bonuses or referral programs as the only solution.
The burden of hiring and training staff
Firstly, even if new site staff are hired quickly, an ongoing effort is required to train new staff in various tasks, including:
- Screening patients that are referred
- Working with often complex digital referral management systems
- Getting up to speed with study protocols and requirements
Given that the effectiveness of, for example, referral screening only increases after a year of experience, current turnover rates mean that hiring new site staff – 54% of whom statistically leave within 2 years – this simply won’t solve the site workload problem.
A recent site survey found that, on average, it takes at least 6 months to hire and train a new staff member who will be dealing with patients. As the survey authors note:
“Sites usually must replace research coordinators with individuals without clinical research experience. This skills gap makes these candidates more expensive to train and oversee. Sites estimate that it takes approximately 10 – 20 weeks to go through the hiring and onboarding process for new patient-facing staff to be able to function quasi-independently, and anywhere from 6 -12 months before they are as fully productive at a capacity equivalent to their predecessor in recruiting and coordinating the existing studies.”
– Society for Clinical Research Sites open letter to sponsors and CRO’s 2022.[5]
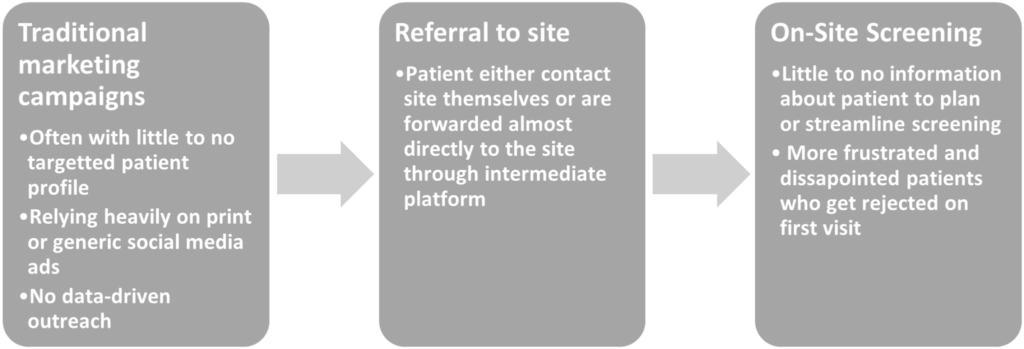
Secondly, even if new staff is hired and trained quickly, this doesn’t change anything to the many of the tasks that cause the high stress, burnout levels and eventual might lead to high turnover rates. Especially screening patients is often a process that takes a lot of time and stress from the staff and at the same time impacts the trial performance and randomization rate.
One important reason that the screening phases place such a heavy workload on site staff is that most sites still use outdated and often overly complicated referral management tools, while screening procedures are often not well defined and an effective, data-driven pre-screening process is lacking. As a result, staff must screen a larger number of patients, a large percentage of whom will not be eligible for the study.
“I was very sad when I learned [at the screening in the research center] that I could not participate in the study. It just felt like you have missed an opportunity again. If you identify someone as me who is willing to participate, but can’t participate at that point in time, they should be given an opportunity later on. If there were a central register, where trials and criteria were shown that would also be a golden opportunity.” – Deborah, Patient’s Voice Participant (request the full Clariness’ Patient’s Voice conference video here).
The result is not just a high workload for the site staff, but as we note in our patient surveys, it also creates frustration and disappointment for patients, who after one bad experience often chose not to participate again in future studies.
Want to learn more about how Clariness’ ClinLife® Platform informs patients about clinical research and helps lower the barrier to participation in Clinical Trials? Request a demo.
The solution to the clinical site staffing and workload problems
As outlined above, the focus on hiring new or additional staff is not a solution to the site workload problem, as other related problems such as on-site screening will remain and continue to result in high turnover rates.
At Clariness, we provide a multifaceted approach to support sites during clinical trials. This starts with a feasibility analysis of the local study population and relies strongly on data-driven outreach methods, a patient friendly digital pre-screener and an easily learned and intuitive referral management system.
Below is an outline of our approach to patient recruitment:
This method is well received by sites across the globe with whom we work, see below a recent piece of feedback from a Clinical Research Study Leader, at a global healthcare company headquartered in Switzerland:
“The combination of recruitment via their online platform and pre-screening at the Clariness Call Center has proved to be a very valuable tool for effectively monitoring, directing and controlling patient referrals to investigator sites. Both our company and the investigator sites are truly satisfied with the service. The high quality of patients referred has resulted in a large number of randomizations and a major reduction in screening efforts at the sites.
Since the start of their participation in the trial, Clariness has referred 55% of all randomized patients — reducing recruitment time by approximately 8 months.”
Here’s how Clariness can help you reduce site staff workload and boost randomizations:
- Data-driven patient recruitment
Our outreach campaigns have the potential to run across more than 40 different channels and are constantly being adjusted based on performance and visitor statistics. Our Patient Insights team builds a patient profile by matching the inclusion and exclusion criteria of the study protocol with data collected over 17 years of patient recruitment. As a result, ads are patient-group specific and only displayed to patients who live within a certain radius of the study site and are pre-screened to meet eligibility criteria. Additionally, online advertising can easily be paused (without the loss of marketing budget) if we predict that screening will be overcrowded. - Innovative pre-screening
Depending on the study and budget, we have either an indication-specific pre-screener questionnaire or a study-specific questionnaire. On the one hand, these help us to sort of filter to make sure that we only refer patients who are likely to be eligible for randomization (using key exclusion criteria questions), but on the other, they also help us to give the research staff more information about the patients at the time of referring them.This then helps streamline the screening process, as site staff can see, for example, which patients still need a biopsy taken and which do not, or when patients are available for contacting. - Phone screeners: Our pre-screener can also be supplemented by a phone screener, facilitated in local language by our in-house, medically trained Enrollment Management Center team. Through this, we obtain additional information from potential study participants and inform them about the study. In this way, participants are more likely to be enrolled in the study and less likely to drop out.
- Referral management tools: Clariness’ Investigator Service is an intuitive referral management tool that helps sites track, contact, and manage referrals in a secure environment. From viewing the answers to online and phone pre-screeners, to making appointments and sending out reminder notifications for visits, the Investigator Service is praised by site staff for its simplicity, extensive options, and data security.
Want to discuss how Clariness can streamline your referrals and screening processes?
To discuss your enrollment pain points, or to get a demo of our patient recruitment platform or Investigator Service, contact us here.
With 20+ years of experience, in which we have worked with 8000+ research sites, successfully supported over 1,200+ clinical trials, and worked with 13 of the 15 largest pharmaceutical companies in the world, Clariness has a thorough knowledge of what a study needs to become a success.
In our latest whitepaper we for example took an in-depth look at other measures sponsors and study sites can take to reduce the chance of clinical trials being delayed. Download it for free here.
Download our site success stories
Clariness has worked with >7,000 sites across the globe and developed teams, solutions, and relationships to directly tackle site burden and increase patient recruitment and retention. Download our success stories below, where we highlight some recent site successes, including:
- Increased site screening per month by 97% and contributed 37% of randomized patients
- Completed recruitment 3 months early through working with 180 sites across 11 countries
- Provided site selection and international recruitment, and contributed 30% of randomizations
- We were given 25% more sites than planned due to successful relationships and performance
___________________________________________________________________________________
[1] https://medcitynews.com/2022/05/what-the-great-resignation-means-for-clinical-research/ and https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2775923
[2] https://medcitynews.com/2022/01/a-whopping-majority-of-surveyed-workers-at-hca-say-staffing-shortage-is-compromising-patient-care/ and https://www.clinicalleader.com/doc/what-s-really-behind-the-cra-shortage-in-clinical-trials-a-root-cause-analysis-0001 https://www.socra.org/blog/career-progression-in-clinical-research/ https://medcitynews.com/2022/02/how-hospitals-can-address-provider-burnout-and-staffing-issues-through-digitization/