How new digital methods can increase atopic dermatitis screener completions and enrollment
Getting ahead of the growing competition for eligible patients with atopic dermatitis
Atopic dermatitis is one of the most prevalent inflammatory skin diseases, with the prevalence and incidence of atopic dermatitis increasing over the past several decades. The lifetime prevalence of atopic dermatitis is 10–20% in children and 1–3% in adults, making it the 15th most common nonfatal disease, and the skin disorder with the highest disease burden in terms of disability-adjusted life-years. Additionally, atopic dermatitis affects subpopulations differently; for example, in the United States, the prevalence of atopic dermatitis is higher among Black/African American children 19.3% compared to White/Caucasian children 16.1%.
For these reasons, scientists and medical experts have increasingly focused on researching atopic dermatitis – and yet, no cure exists, and those with mild-to-moderate atopic dermatitis still lack effective therapeutic options. This focus has led to the increasing number of dermatological clinical trials we see today; at the time of writing this, there are currently 500+ dermatological trials underway. As one of the primary causes of clinical trial delays include patient recruitment, screening, and retention, it is not difficult to imagine how fierce the competition is for eligible patients with this number of trials in operation.
Atopic dermatitis screener customization and innovation is a necessity in the digital world
COVID-19 caused a decline in clinical trial enrollment, with trials being halted or discontinued during the pandemic. With the rapid increase in internet usage, digital patient screening is no longer a future scenario, but the reality of today’s patient recruitment. At Clariness, our pre-screening methods, protocol, and optimization of screening techniques are customized to meet each clinical trial’s specific needs, thereby contributing to overall success.
Patient recruitment is one of the biggest causes of clinical trial delays, with 40% of clinical trials experiencing delays due to poor patient retention and 30% of study terminations due to enrollment difficulties. Researchers have argued that manual screening for clinical trials is a laborious and inefficient process. Moreover, researchers suggest that increased usage of electronic screening can potentially identify more eligible patients for clinical trials.
Having successfully performed patient recruitment for 20+ international atopic dermatitis trials, and being one of the early initiators of electronic screening techniques, we can confidently agree with these researchers.
Clariness’ innovative handprint BSA screening method
Understanding the barriers to atopic dermatitis patient recruitment and the impact it can have on trial timelines, we use digital and innovative techniques to break through recruitment barriers. One of the key eligibility criteria for almost all atopic dermatitis clinical trials is knowing the body surface area (BSA) affected by the disease, which, has traditionally been difficult for patients to accurately self-identify. As such, Clariness developed a unique handprint screening technique to quickly identify eligible patients ahead of referring them to sites, and thus increasing patient enrollment numbers.
This BSA screening technique allows patients to better self-identify symptom severity during the completion of both phone and online screeners, with a customizable threshold adapted for screening as per the trial protocol’s requirements.
How the handprint BSA technique works
A patient uses their handprint to self-identify the affected BSA and reports this as part of Clariness’ screening process. Estimating how much BSA is affected is an important score to assess disease severity and a fundamental element of atopic dermatitis clinical trial inclusion and exclusion criteria. To estimate the body surface area, the number of palms of one’s hand covering the body is used. A cutoff of 10% or higher is commonly used as an indicator of moderate to severe atopic dermatitis in clinical trials.
Below is an example of how the BSA screening technique is used: in this scenario, the patient has self-identified a BSA of 4% (Figure 1). To the right, based off a survey with >7000 respondents, we see how often patients reported a certain body area as affected by their atopic dermatitis.
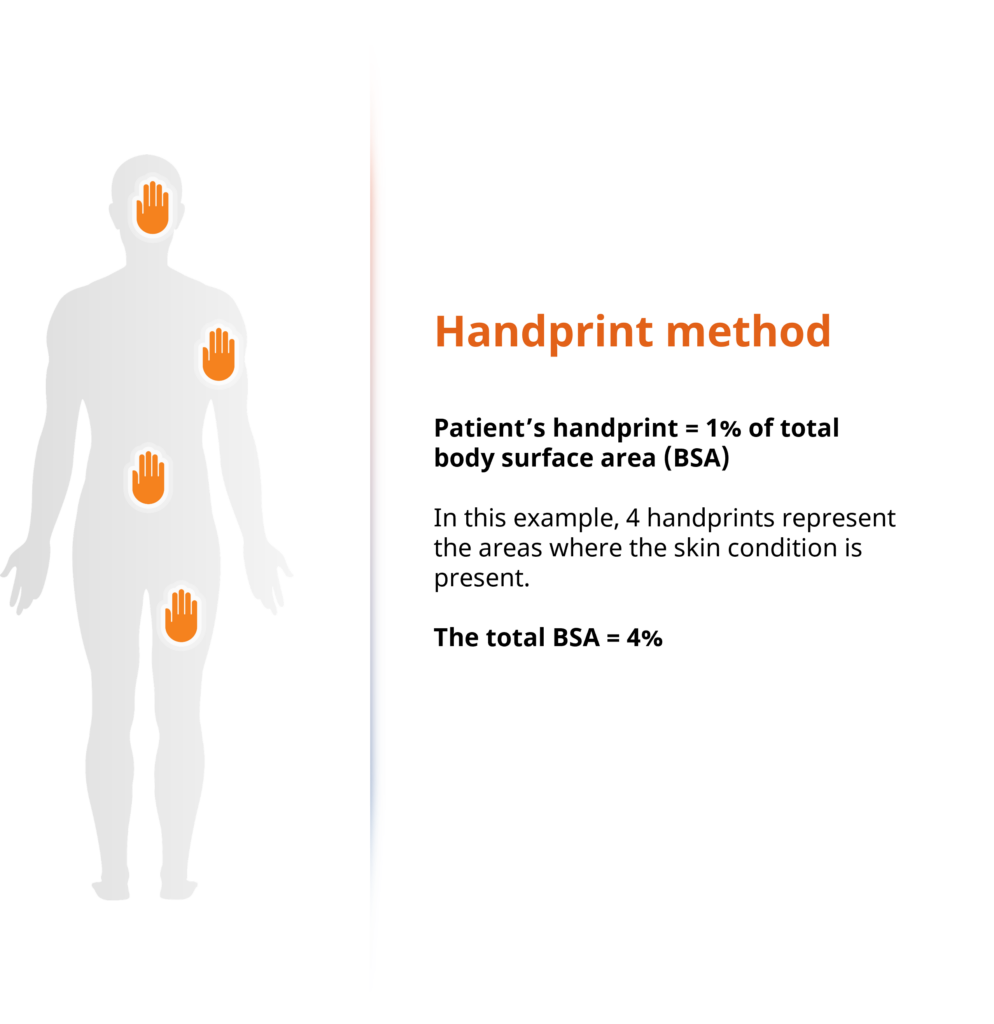
Figure 1: Clariness’ innovative handprint BSA screening method.
This innovative, yet simple screening technique has enabled Clariness to:
- Ensure that more patients qualify when referring them to sites
- Limit study delays by ensuring more patients are consented to and randomized
By using Clariness’ BSA screening technique, patients can self-identify their affected BSA by simply using their hands. This benefits patients as they can self-assess at home and know if they are eligible for the study before traveling to a site. This also allows for the evaluation of symptom severity, before being referred.
The benefits of leveraging digital recruitment and screening techniques
By continuously optimizing and customizing digital outreach and screeners, we at Clariness have seen an increase in recruitment campaign performance, in the form of completed patient screenings and enrollment numbers of both adults and adolescents.
The customization and innovation of our screeners are the results of continuously optimizing them using the best performing adaptations from 10+ years of digital atopic dermatitis screenings. Dermatology research has been one of our fields of expertise for over a decade, we have digitally recruited and screened patients with atopic dermatitis for 20+ dermatology studies worldwide. In doing so, we’ve learned how to optimize and innovate screeners across inclusion and exclusion criteria, medical history, diagnosis, and more. We also leverage a 2-step screening method (online and telephone screening), to ensure higher numbers of patients are both referred and enrolled by sites.
Below is an example of a recent atopic dermatitis campaign (Figure 2), which shows how our digital recruitment and screening methods drove both adult and adolescent patients to our landing pages, through our screening steps, and successfully passing them. Using a 2-step screening approach (online and phone screening), we consistently register and refer a high number of patients.
Figure 2: Overall pre-screening results for both adults and adolescents.
Clariness has successfully implemented a thorough pre-screening process for caregivers and adults consisting of online and phone screening to select only the most eligible adult and adolescent patients. These results prove that tailoring patient recruitment strategies and continuously optimizing screening methods at every step has boosted and increased the engagement and enrollment of patients with atopic dermatitis.
Learn more about Clariness’ atopic dermatitis patient recruitment
To learn more about our patient recruitment expertise within atopic dermatitis, screening methods, and our digital techniques – visit our atopic dermatitis page. To speak with one of our patient recruitment experts, click here.
Want to know how we can support your atopic dermatitis patient recruitment?
Simply get in touch with one of our experts, and we will review your study’s requirements, and develop a strategy to enroll more patients.
Insights and recourses



