Exploring design and terminology in clinical trials: Which images and content resonates more with patients?
By Joseph Yeomans, VP Marketing, Clariness
The importance of patient preferences
Patient preferences should be at the center of clinical trial design, from the design of the trial itself, down to the design of the study materials that patients see and use. At Clariness, we have surveyed over 1,000,000 patients, and every year we drive 15,000,000 patient visitors to our patient portal, ClinLife®. Gathering data and input from this many patients on a global scale provides Clariness with unrivaled insights into the most effect designs for study materials and recruitment campaigns.
By understanding patient preferences in the creation of both study materials and recruitment campaigns allows sponsors to reach and engage larger groups of their target population, by knowing which imagery, content, channels, and medical detail resonate most with patients.
Data-driven patient preferences
We recently conducted a survey with 268 patients in 7 different countries (South Africa, United States, Canada, Germany, United Kingdom, Poland and Korea) to better understand the preferences of patients in terms of material design, terminology and technical terms for clinical trials.
Specifically, the survey looked into:
- Image preferences e.g. Whether to show the condition or not, animated or live action
- Preferred colors e.g. what emotions are triggered, does the indication have a suited color
- Terminology and verbiage e.g. Technical vs. non-technical terms, preferred ‘labels’
In this short blog, we highlight some of the survey’s findings that you can take as learnings for future material design.
Patient preferences on study material imagery
We presented patients with example images that could be used within study and advertising materials, requesting patients to opt for their preferred images. Over three such activities, we summarized the results to find that an overwhelming 85% of patients preferred real images vs. illustrations (cartoon images), as seen in Figure 1.
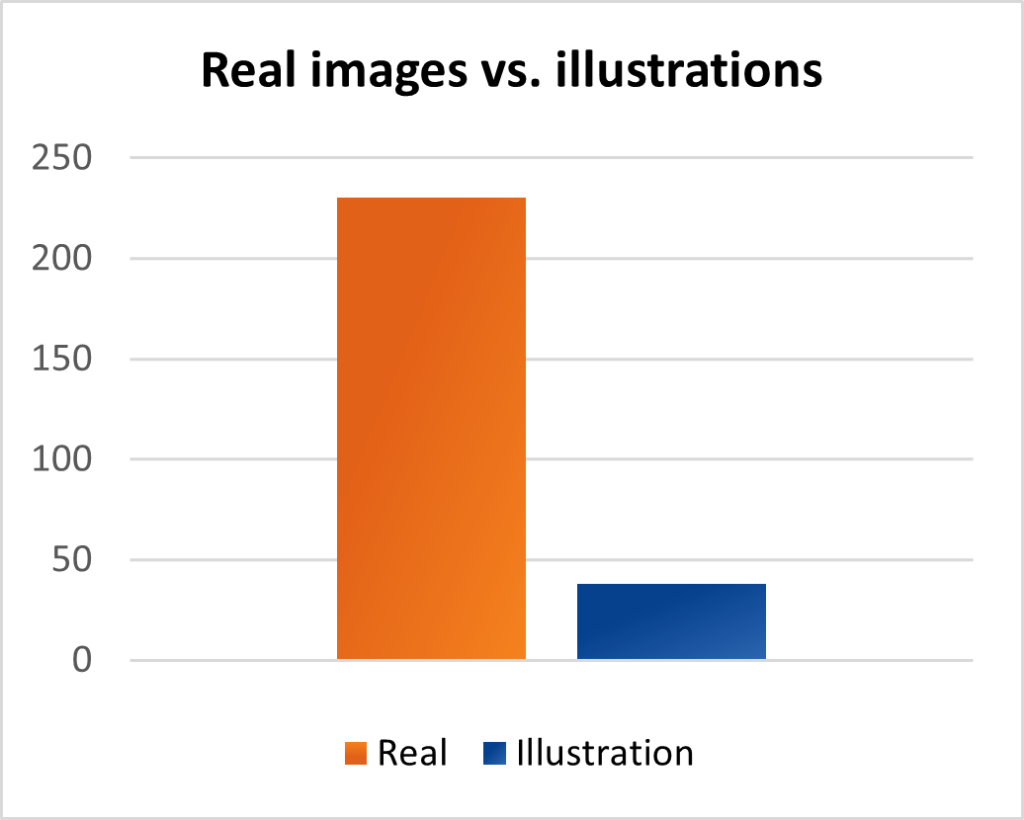
Further, when questioned on the subject of the images being shown in these materials, patients opted to see images showing the indication, even patients suffering from the condition. The results can be seen below:
- Showing the indication – 69%
- No faces being shown – 21%
- Happy patients – 17.6%
- Neutral response – 17.6%
- Scenic imagery – 11%
Note: Patients could choose multiple options
Both of these results show that patients want to see real people, dealing with the condition they have, which suggests patients want relatable images. Consider this in your future material designs, to include images of patients who resemble your target population, and accurately depict the condition.
Patient preferences on study material content
Surveying global patients on the content they would most want to see in study materials, revealed that patients are more interested in the benefit of the study for themselves and the community, than side effects and study requirements.
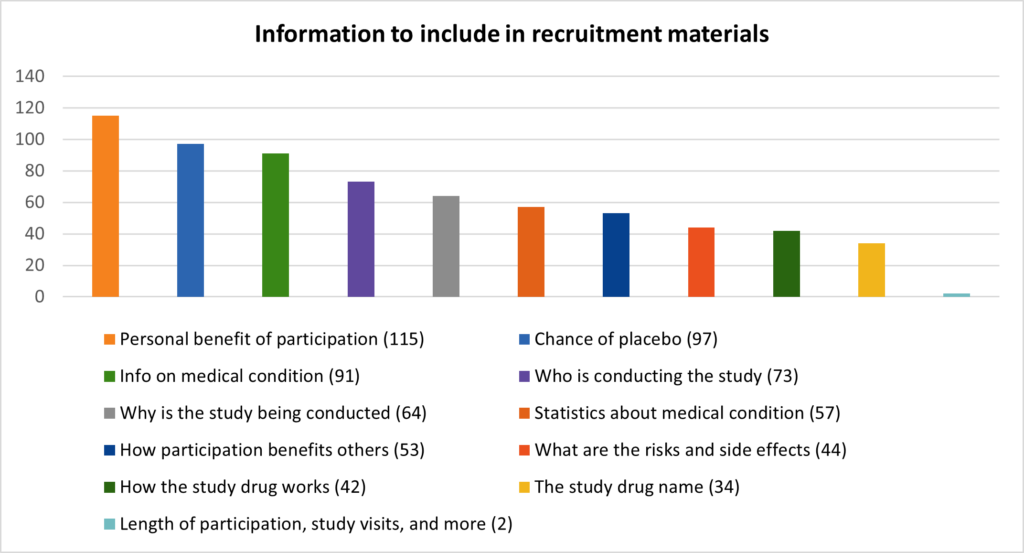
In the data below seen in Figure 2, we ranked the priority of information types wanted within clinical trial study information.
The 3 most desired information types:
- Personal benefit of participation
- Chance of receiving a placebo
- Information on the patient’s medical condition
The 3 least desired information types:
- Length of participation, number of study visits and more
- The name of the study drug
- How the drug works
While this data would suggest that study requirements and risks of side effects are less important to include in study information, it must be considered that these results are from patients considering top-level, initial study materials.
Considering this, it is important to convey altruism as a driver of participation, stating immediately whether the study has a placebo control, and provide information and stats regarding the condition being studied. This will peak patient interest on a global level, according to our data.
Patient preferences on medical detail of study materials
Now that you aware of the information to include, you must decide how technical that information should be, and should this vary depending on the country, indication and education levels of your target patient population. In short, there are many variables surrounding the level of medical detail your study materials should delve into, however, our findings on a global level, suggest that medical information should be simplified, regardless of whether the patient has a medical or scientific background.
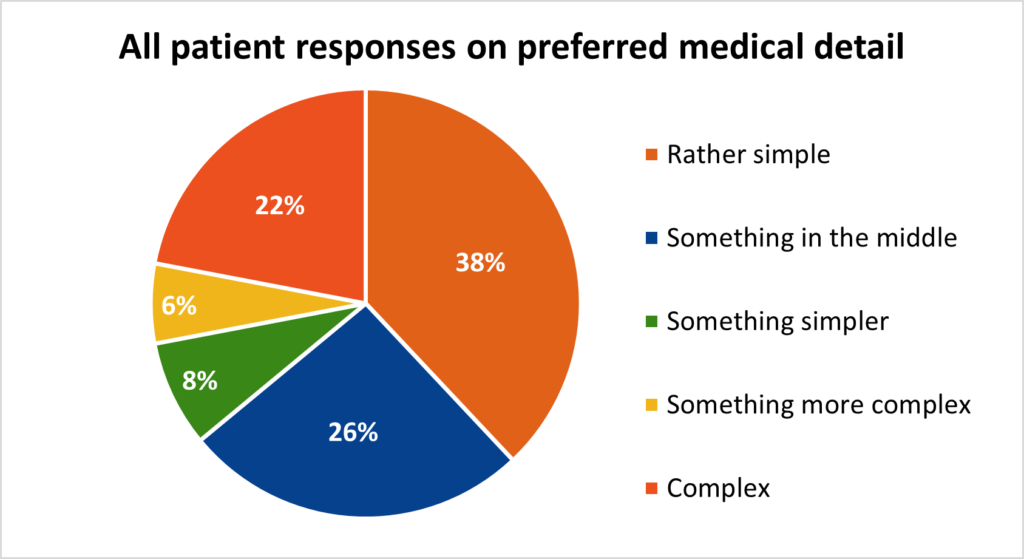
While the data shows that there’s still a significant number of patients who would appreciate more complex medical detail, the majority have a preference for more straightforward explanation, and moving toward this end of the spectrum will be more inclusive of the wider patient population.
Furthermore, individuals seeking in-depth medical information can engage in discussions with their medical practitioner or visit a study website that can satiate that need for more detail.
How can you leverage these patient preferences?
While these data provide a general indicator on patient preferences as a whole, it is important to note that all patient populations are unique, not only by an indication, but the variables of location, age, gender identity, education level, insurance coverage and many more.
These variables influence the sources that patients trust, how they wish to access and digest content, and their personal drivers to trial participation. At Clariness, we suggest accessing the patient’s voice for all trials, not only to obtain study preferences, but to pre-fill patient pipelines as we can match respondents to the I/E criteria of your study.
To find out more regarding how we can provide patient insights and tailored recruitment campaigns that resonate with your target population, anywhere in the world, please reach out to one of our experts.
Want to learn more about our patient insights?
Insights and recourses



