Patient and site insights designed to improve your study’s recruitment
We gather insights directly from patients and HCPs across the globe, provide our key findings, and organize the data in our Clinlytics platform.
These insights answer the questions your study team should be asking:
- How does your study’s target population vary by country, ethnicity, and more?
- What is the trade-off between your I/E requirements and patient willingness to participate?
- Do your chosen sites have the right patients, technology, and staff to support your study?
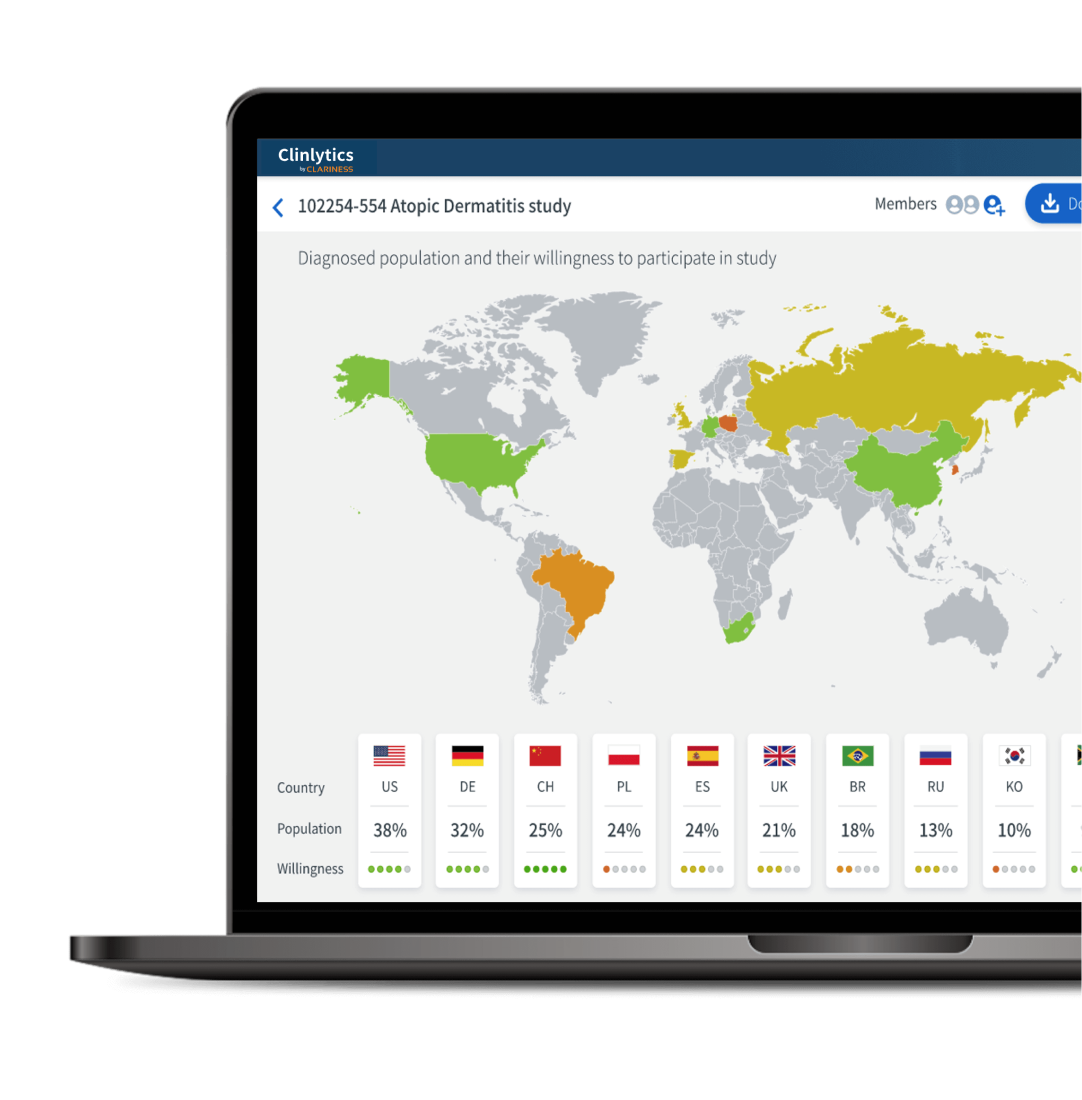
Our insights identify avoidable costs & delays
By identifying recruitment barriers, optimizing protocols, and selecting the most appropriate sites, we enable sponsors to conduct more efficient, patient-centric clinical trials.
–
Insights aligned to your focus areas
If you’re seeking to enhance your understanding of the target patient population, refine site selection, optimize protocols, or explore important focus topics such as clinical trial diversity, we can support you and your study.
Patient focus
- Identify biggest barriers for your patient population
- The patient-reported impact of their condition
- Study participation vs. condition severity
Geography focus
- Choose sites with the right staff & patients
- Which countries host your target population
- Identify site locations with more diverse patient pools
Topic focus
- Design a study that boosts diversity, equity, & inclusion
- Learn how DCT can benefit your studies
- Create new study evaluation processes
Protocol focus
- Pre-empt expensive protocol amendments
- Know which procedures are truly required
- Have patient feedback on your I/E criteria
Why choose Clariness for feasibility?
Design more patient-centric studies
Feasibility & performance data from 1,500 studies
We leverage feasibility data from 20+ years of supporting studies across 175+ indications
Pre-populate your patient funnel
Medical experts & trained psychiatrists
Pre-identify costly amendments
Contact us
Get in touch to find out how we can accelerate your clinical trials
Speak to one of our experts today for personalized solutions and support
